About microRNAs.
We believe that targeting pathways of human disease with antisense oligonucleotides targeting RNA biology represents a novel and potentially powerful therapeutic approach for multiple diseases.
We are beginning this work with therapies designed to target microRNA regulation of protein expression. MicroRNAs are small, naturally occurring, non-coding RNAs that function as important regulators of gene expression of messenger RNA (mRNA) and play a role in multiple cellular processes. They do this by binding specific mRNA and blocking translation, leading to control of gene expression and direct degradation of target mRNA.
These naturally occurring non-coding RNAs were first discovered in 1993, and have been identified in almost every mammalian species, including humans. This conservation across species supports the important role of these regulatory RNA elements in mammalian biology.
MicroRNAs play an integral role in numerous biological processes, including the immune response, cell-cycle control, metabolism, viral replication, stem cell differentiation, and human development. There are about 2,600 predicted mature human microRNAs, 600 of which are considered to be well validated and provide a large space of novel target opportunities. Indeed, microRNA expression or function is significantly altered in numerous diseases including cancer and fibrosis, as well as in CNS, metabolic and inflammatory disorders.
We are currently focused on genetic kidney diseases, where we have evolved our foundational technology and demonstrated the ability to preferentially distribute our therapeutic molecules to the kidney in both preclinical models and in patients.
Mechanism of action.
Pipeline.
Our clinical pipeline centers on orphan kidney diseases of high unmet need, including Autosomal dominant polycystic kidney disease (ADPKD).
Our ADPKD product candidate, RGLS8429, is being evaluated in a Phase 1b clinical trial. (NCT05521191)
About ADPKD.
ADPKD is an orphan disease of high unmet need that effects approximately 160,000 diagnosed individuals in the U.S.
Total Patient Population
-
12M
Globally
-
500K
In the U.S.
of patients develop end stage renal disease by age 60 and require dialysis or transplantation
The only existing FDA-approved agent for ADPKD, tolvaptan, carries a boxed warning for potential fatal liver injury
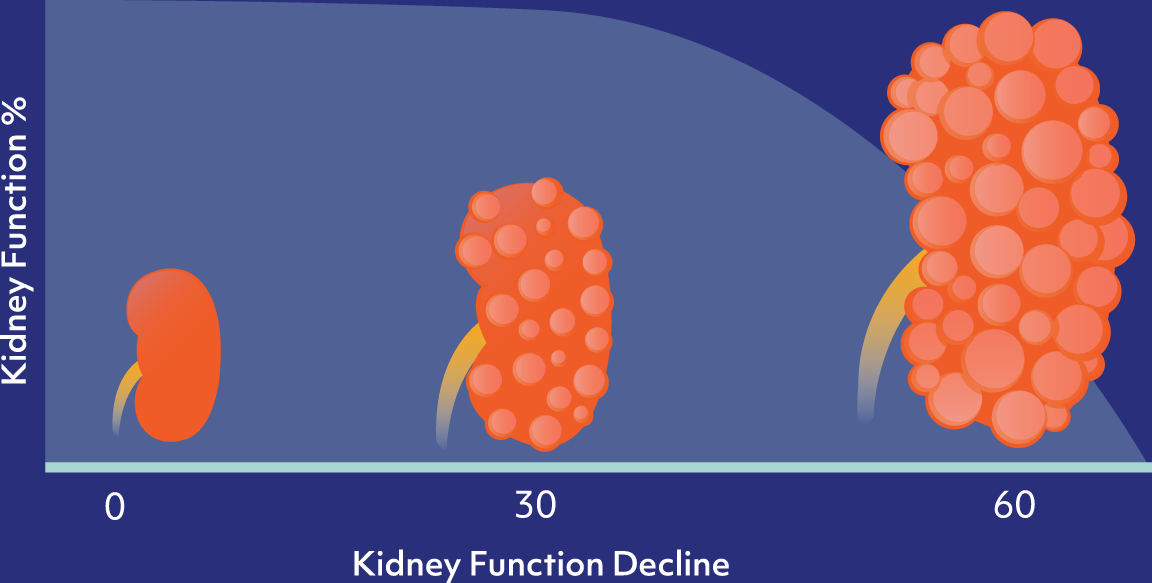
CAUSES
ADPKD is caused by a mutation of either the Pkd1 or Pkd2 genes, which leads to the formation and proliferation of fluid-filled cysts in the kidneys, and ultimate loss of kidney function over time.
About RGLS8429
We are advancing our next-generation, anti-miR-17 compound, RGLS8429, to treat ADPKD. Our work in this disease area builds on the positive clinical results seen with our first-generation compound. RGLS8429 is supported both by robust data in preclinical models, where we have seen clear improvements in kidney function, size, and other measures of disease severity, as well as a superior pharmacologic profile.
Notably, we have also seen additive efficacy in animal models of ADPKD when combining our first-generation ADPKD candidate with the only FDA-approved agent for the disease, tolvaptan, an important commercial consideration for those that can tolerate tolvaptan.
RGLS8429 Mechanism of Action
Pkd1 or Pkd2 gene mutations are associated with an increase in the expression of a specific microRNA (miR-17), which directly leads to the repression of its target genes and as a result, a decrease in the proteins they encode, polycystins 1 and 2 (PC1 and PC2).
RGLS8429 Mechanism of Action
We believe treating ADPKD with an anti-miR-17 therapy will correct the underlying pathology of ADPKD by inhibiting miR-17 function in the kidney.
RGLS8429 Mechanism of Action
miR-17 inhibition will enable increased translation of target mRNA.
RGLS8429 Mechanism of Action
…leading to increased PC1 and PC2 protein levels and reduction of cyst growth.
